Choosing an
alternative path
to current nasal polyp
treatment options with
SINUVA 
Which of your patients may benefit from SINUVA?10
Patients ≥ 18 years of age who:
Had previous ethmoid sinus surgery
Have CRS with nasal polyps

Patients who
meet these requirements and desire an alternative to revision sinus surgery
to manage their worsening symptoms may find relief with SINUVA


About Nasal Polyps The road to symptom relief can be difficult

Nasal polyps develop by intrinsic mucosal inflammation that persists in the ethmoid sinuses1
Symptoms include:
Recommended treatment options for patients with nasal polyps include saline nasal rinses, topical intranasal and oral corticosteroids1
Challenges of current
recommendations
Topical intranasal corticosteroids
-
~60% of active drug in a metered dose of nasal steroid spray is removed by mucociliary clearance within 15 minutes2
-
Only 32.7% of patients use intranasal corticosteroids as directed3
Saline nasal rinses
-
Compared directly with topical nasal steroids, the benefits of saline irrigation alone are less pronounced1
Oral corticosteroids
-
Regardless of dosage and length of treatment, oral corticosteroids carry known safety concerns associated with chronic systemic exposure4
UNDERSTANDING
the cyclical nature
of nasal polyps
Patients who fail to achieve symptom relief with medication management turn to endoscopic sinus surgery (ESS)

Patients with nasal polyps require revision ESS more frequently than patients who do not have nasal polyps5,6

>0,000 ESSs
are performed in the US
annually7
Challenges of sinus surgery
ESS for polyp removal is associated with higher blood loss than nonpolyp sinus surgery, with blood loss impairing visualization resulting in a riskier procedure5
Surgical landmarks are often absent or distorted in revision ESS, making surgery more challenging and increasing risk for patients5
Despite the use of steroids and endoscopic sinus surgery, recurrence of nasal polyps is common due to the inflammatory nature of the disease, resulting in the need for repeat surgery in patients over time5

Key benefits of SINUVA
SINUVA is a novel implant with a 2-in-1 mechanism that offers patients with nasal polyps symptom relief via:
-
Targeted delivery of 1350 µg of mometasone furoate to the ethmoid nasal polyps10
-
Self-expanding, bioabsorbable design that softens over time10
Continuous delivery of mometasone furoate over the course of 90 days10
Localized drug delivery minimizes reliance on patient compliance
Administered in a non-surgical, in-office procedure with local anesthesia10
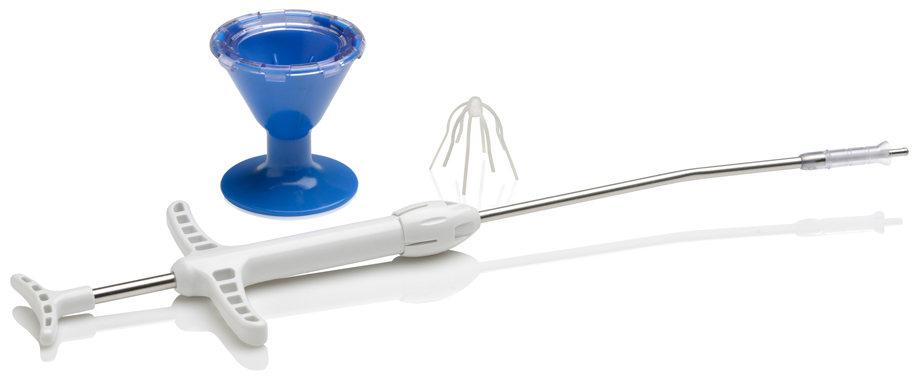
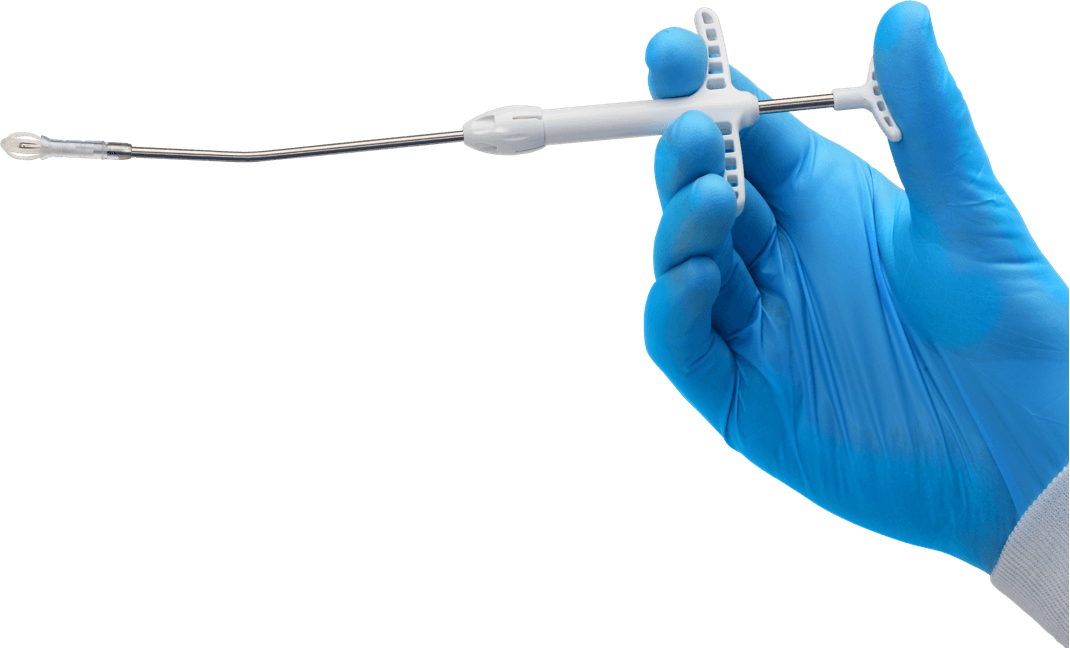
Design of SINUVA10
Width when crimped: 7.5 mm
Nominal length
of 20 mm
SINUVA (7.5 mm)


Dress Shirt Button (7 mm)
Why mometasone furoate compared to alternative corticosteroid options?
Mometasone furoate offers high potency and low systemic bioavailability11
lipophilicity
(absorption into tissue)
glucocorticoid potency
(remains in the tissue)
systemic bioavailability
(nominal systemic absorption)
How does SINUVA work?10
SINUVA is implanted in your
sinus cavity amongst the nasal polyps to provide local delivery of a drug
called mometasone furoate (a type of steroid). The implant has been shown to
be effective in reducing symptoms nasal obstruction/
SINUVA creates
a path to improvement
by demonstrating positive
efficacy data
Co-primary efficacy outcomes10,12,13*†
In RESOLVE II (n=300), SINUVA patients experienced statistically significant reductions in bilateral polyp grade and nasal obstruction/congestion score
0
relative improvement in bilateral polyp grade from baseline to Day 90 compared to once daily mometasone furoate nasal spray alone
-
Mean change (SD) for SINUVA –0.56 (1.06) vs –0.15 (0.91) with control; P=0.0073
0
relative
improvement in nasal
obstruction/
-
Mean change (SD) for SINUVA –0.80 (0.73) vs –0.56 (0.62) with control; P=0.0074
*Patients in the SINUVA group also received once daily mometasone furoate nasal spray daily.
†Co-primary endpoints: Change from baseline to Day 90 in bilateral polyp grade, as determined by an independent panel on a scale of 0 (no visible nasal polyps) to 4 (nasal polyps completely obstructing nasal cavity). Change from baseline to Day 30 in nasal obstruction/congestion score, as determined by patients on a scale of 0 (no symptoms) to 3 (severe symptoms).10
Secondary efficacy endpoints (baseline to Day 90)12,13‡
Reduced eligibility for repeat surgery
-
SINUVA placement resulted in a 61% reduction in the proportion of patients still indicated for repeat ESS vs 37% in the mometasone furoate nasal spray only arm at Day 90 (P=0.0004)
Delivered up to 90 days of sustained symptom relief
-
Patients treated with SINUVA experienced sustained symptomatic improvements in nasal obstruction/
congestion at Day 90 -
Mean change (SD) for SINUVA –0.93 (0.80) vs –0.69 (0.79) with control (P=0.0248)
Significantly reduced sinus obstruction
-
Patients treated with SINUVA had a significantly greater decrease in percent ethmoid sinus obstruction at Day 90
-
Mean change (SD) for SINUVA –11.3 (18.1) vs –1.9 (14.4) with control (P=0.0007)
Improved patients' sense of smell
-
Patients treated with SINUVA experienced a significant improvement in their self-reported sense of smell score on a six-point Likert scale at Day 90
-
Mean change (SD) for SINUVA –1.20 (1.66) vs –0.76 (1.60) with control (P=0.0470)
Patients treated with SINUVA did not experience a significant improvement in their self-reported facial pain and pressure score. The mean change (SD) for SINUVA was –0.77 (1.21) vs –0.90 (1.27) with control (P=0.9130)
SD, standard deviation.
‡Secondary
endpoints: Proportion of patients still indicated for repeat ESS at Day 90
despite ongoing intranasal steroid use based on clinical investigator
assessment using study-specific criteria. Change from baseline to Day 90 in
nasal obstruction/
RESOLVE II: Study design10,12
Randomized, controlled, double-blind, multicenter study with 300 patients
Study population:
- ≥ 18 years of age
- Diagnosed with chronic sinusitis
- Had undergone prior bilateral total ethmoidectomy
- Indicated for revision endoscopic sinus surgery
- Presented
with moderate to severe nasal obstruction/
congestion symptoms and recurrent bilateral sinus obstruction due to sinonasal polyposis despite the use of intranasal corticosteroid sprays and recent high-dose steroids

201 patients were randomized to the SINUVA treatment arm where they underwent bilateral placement of SINUVA in the ethmoid sinuses
99 patients were randomized to the control arm where they received a sham procedure
Patients in both study arms received once daily mometasone furoate nasal spray (200 µg) through day 90

SINUVA safety data
Adverse reactions with > 1% incidence rate and more common in treatment than the control group in combined data from Study 1 and Study 2
| ADVERSE REACTIONS | SINUVA (n=254) n (%) | CONTROL (Mometasone furoate nasal spray) (n=146) n (%) |
|---|---|---|
| Asthma | 12 (4.7) | 6 (4.1) |
| Headache | 9 (3.5) | 5 (3.4) |
| Epistaxis | 6 (2.4) | 2 (1.4) |
| Presyncope | 6 (2.4) | 3 (2.1) |
| Bronchitis | 5 (2.0) | 2 (1.4) |
| Otitis media | 5 (2.0) | 2 (1.4) |
| Nasopharyngitis | 3 (1.2) | 1 (0.7) |
In clinical trials, SINUVA demonstrated similar local effects and hypersensitivity reactions compared to once daily mometasone furoate nasal spray alone, with a low incidence of serious adverse events10,12
-
Patients on SINUVA did not experience any significant increase in intraocular pressure, glaucoma, or any form of cataract10
Placement of the SINUVA Sinus Implant10
Placement of the SINUVA Sinus Implant10
The SINUVA Sinus Implant is loaded into a Delivery System and placed in the ethmoid sinus under endoscopic visualization. For the complete instructions on how to place SINUVA, see the Full Prescribing Information.

The SINUVA Sinus Implant is made from bioabsorbable polymers designed to gradually soften over time. The implant was designed to gradually release the corticosteroid directly to the nasal polyps over a period of 90 days. Remove the SINUVA Sinus Implant at day 90 or earlier at the physician’s discretion using standard endoscopic instruments.10










Preparing your patient for implantation of SINUVA10,12

The patient should be prepared following routine protocols for in-office sinonasal endoscopic procedures.
In the clinical study, topical or local anesthetic—such as sprays, pledgets, and cottonoids—was recommended in accordance with protocols for in-office sinonasal endoscopic procedures.
In the clinical study, anesthesia injections were used as necessary.
Note: Patients cannot remove SINUVA themselves. Advise the patient that the implant is bioabsorbable and intended to soften over time. Remove the implant at day 90 or earlier. As the implant softens and polyps decrease, the implant may be expelled out of the nose. If they experience excessive bleeding, pain, or a choking sensation in the throat, they should contact a physician immediately.
Patient Resources
The following resources can be used to provide your patients with information on SINUVA:
Ordering
SINUVA
SINUVA implants can be obtained through two accessible avenues:
Specialty Distributor: providers can purchase SINUVA implants directly from a distributor listed below. If use is for a specific patient, obtaining prior authorization from the payor is recommended prior to ordering.

1-855-571-2100 (McKesson Med-Surg)
For Physician Offices

1-800-543-2111
For Facilities

1-800-746-6273
Specialty Pharmacies: SINUVA implants can be filled with a prescription at select specialty pharmacies and delivered directly to the provider.

1-800-356-4354

1-833-858-7788

1-855-244-2555
The Connect Support Program can assist with the acquisition process by researching and verifying patient insurance benefits for SINUVA.
To utilize this service, complete the
Patient Enrollment Formand fax it to 1-844-745-2358.
For questions,
call Connect directly at 1-833-4-SINUVA (1-833-474-6882)
Connect Program OverviewCoding
SINUVA
Effective April 1, 2021
The Centers for Medicare and Medicaid Services (CMS) has made the following changes to the HCPCS code set:
New J-Code for SINUVA
(Mometasone furoate sinus implate, 1350 mcg):J7402
"Mometasone furoate sinus implant, (sinuva), 10mcg"| ICD-10 CODE | DESCRIPTOR |
|---|---|
| J33.0 | Polyp of nasal cavity |
| J33.1 | Polypoid sinus degeneration |
| J33.8 | Other polyp of sinus |
| J33.9 | Nasal polyp, unspecified |
| NDC NUMBER | DESCRIPTOR |
|---|---|
| 10599000301 | SINUVA (mometasone furoate) sinus implant |
| HCPCS CODE | DESCRIPTOR |
|---|---|
| J7402 | Mometasone Furoate sinus implant, 10 micrograms |
| Ambulatory Surgery Center/Hospital Outpatient Surgery Code | DESCRIPTOR |
|---|---|
| J7402 | Mometasone Furoate sinus implant, 10mcg Pass-through status code for use with Fee For Service Medicare patients only |
Billing units for J7402 is 1 unit for every 10mcg = 135 units for unilateral placement of SINUVA or 270 units for bilateral placement of SINUVA.
When billing Medicare with J7402, include the NDC number (10599-0003-01) on the claim form along with the CPT code to describe the services provided.
SINUVA should not be billed directly by a provider if it is distributed via specialty pharmacy. Providers should always bill the most applicable CPT code for the procedure performed at the time of placement of SINUVA.
Codes listed above are recommendations and do not guarantee payment or reimbursement.
For additional SINUVA reimbursement support, please contact reimbursement@intersectent.com.
For further information on the price of SINUVA, please contact Intersect ENT at 1-866-531-6004.







