Did You Know?
Nasal polyps can resurface after sinus surgery, like weeds after removal
Why do nasal polyps come back like weeds after sinus surgery?
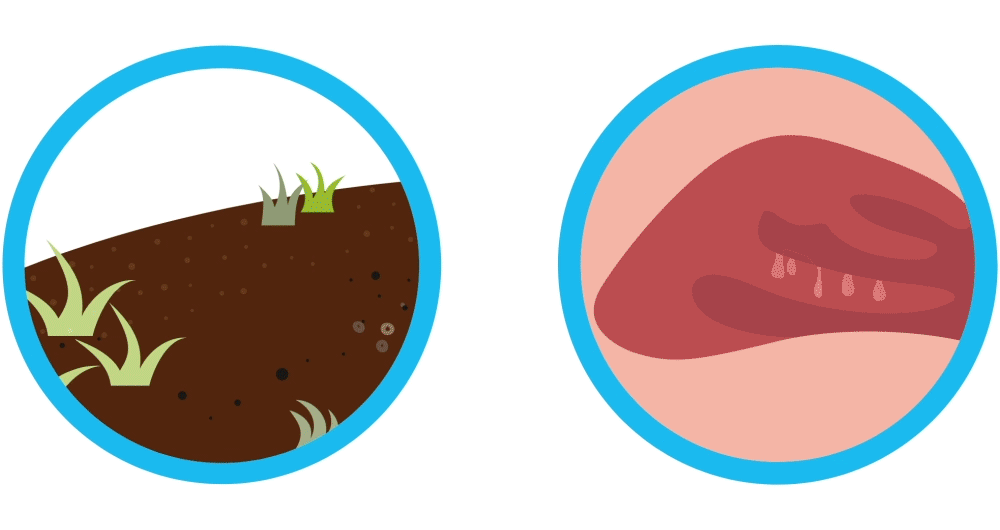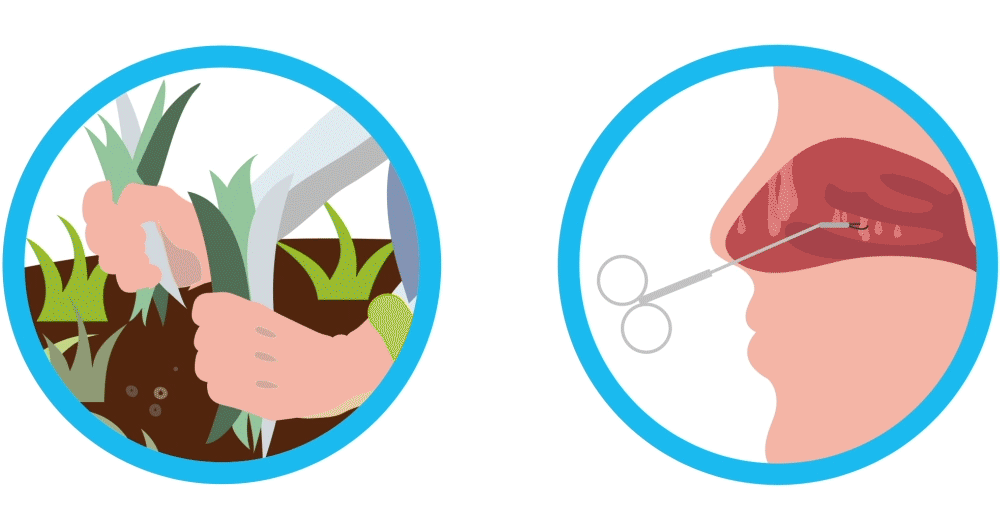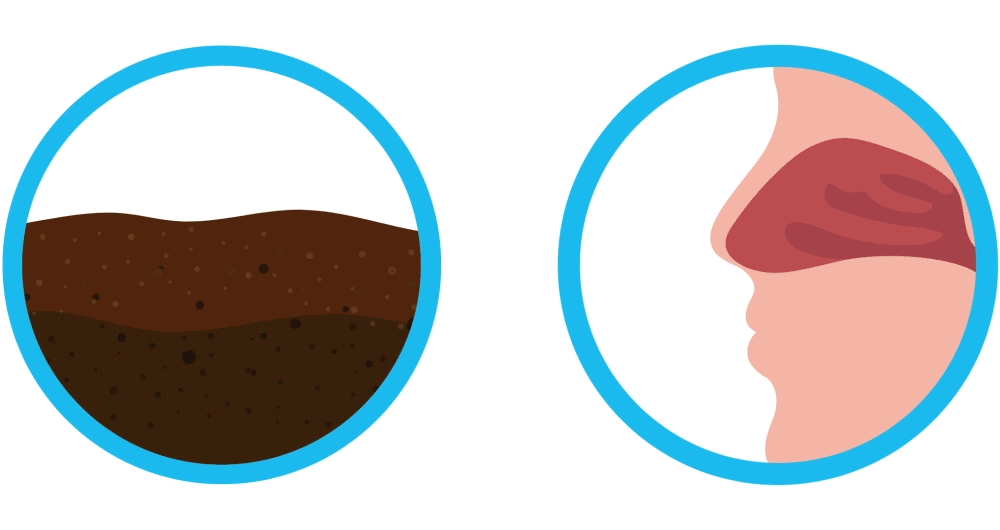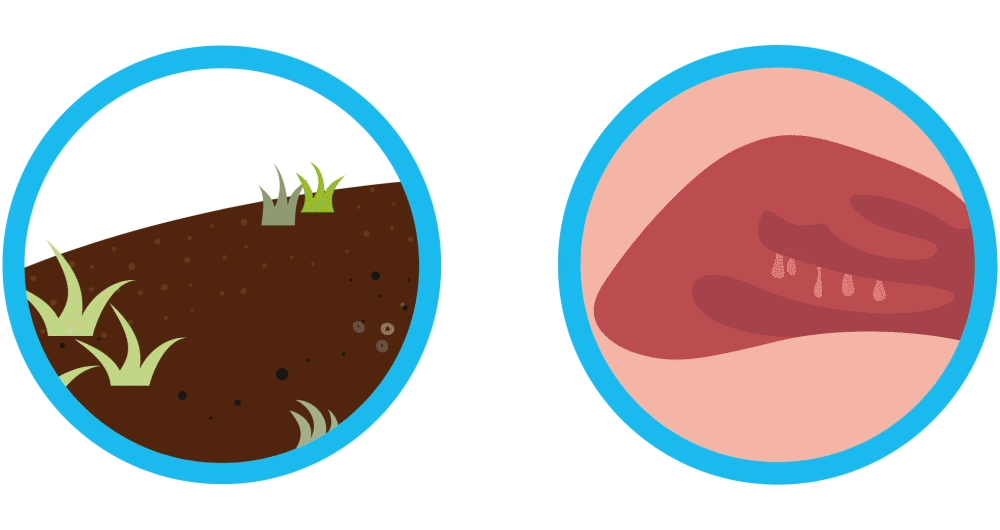
Imagine your nasal polyps are like weeds. You remove the weeds, but they may grow back again. Controlling weeds may require treatment once they’ve grown back, and nasal polyps are similar. Nasal polyps are soft, non-cancerous (also called “benign”) growths that develop as a result of chronic sinus inflammation.
Nasal Polyps come back in 1 out of 3 patients at 6 months after sinus surgery.
See how Dr. Robert Kern Describes Recurring Nasal Polyps
Nasal polyps are soft, non-cancerous (also called “benign”) growths that develop as a result of chronic sinus inflammation. Symptom relief often requires sinus surgery after medicines fail. Even though sinus surgery helps remove nasal polyps, sinus surgery is not a cure and your nasal polyps may pop up again. In fact, it’s common for nasal polyps to come back and require additional treatments.
See how SINUVA can helpDiscover relief, the SINUVA Way
SINUVA is a treatment to help you manage your nasal polyps that have come back after ethmoid sinus surgery, similar to treating weeds to keep them controlled after they grow back.
With your nasal polyps under control, the goal is to get back to your daily activities

- Breathe easier and enjoy the outdoors, whether it’s gardening or barbeques with your family
- Smell more and enjoy the smell of coffee, freshly mown grass, perfume, and flowers
*Anti-inflammatory medicine (mometasone furoate) was not detected 14 days after placement of SINUVA in a pharmacokinetics study.
Is SINUVA right for you?

SINUVA can help you manage your nasal polyps that have come back after ethmoid sinus surgery.
Key benefits of SINUVA
SINUVA is placed in the sinus cavity during a routine office visit
How SINUVA works
SINUVA’s safety profile has been proven in clinical trials.
Proven safety recordDiscuss the risks of SINUVA with your SINUVA doctor.
INDICATION
SINUVA Sinus Implant is a prescription steroid-releasing implant indicated for the treatment of chronic rhinosinusitis with nasal polyps in adult patients 18 years or older who have had ethmoid sinus surgery.
IMPORTANT SAFETY INFORMATION
Who should not use SINUVA?
Do not use SINUVA if you are allergic to mometasone furoate or any ingredients of the implant.
What should I tell my doctor before receiving SINUVA?
Before you receive SINUVA, tell your doctor about all medical conditions you have including nasal/sinus problems (such as nasal ulcers or trauma), eye problems (such as glaucoma or cataracts), or any untreated fungal, bacterial, or viral infections.
What are the possible side effects of SINUVA?
Serious side effects of SINUVA can include:
-
Local nasal adverse reactions including nosebleed and injury to nerves or blood vessels in the nose/sinus.
-
Serious allergic reactions have happened in patients using mometasone furoate including rash, itching or swelling of the lips, face, tongue, and throat, and breathing problems. Call your doctor right away if you have any of these reactions.
-
Weakened immune system that may increase your risk of infections. Avoid contact with people who have contagious diseases such as chickenpox or measles. Call your doctor right away if you have been near someone with chickenpox or measles.
-
Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones and can cause tiredness, weakness, nausea and vomiting and low blood pressure. Talk to your doctor if steroid effects such as Cushing Syndrome and adrenal suppression appear.
The most common side effects of SINUVA in clinical studies were bronchitis, cold symptoms, middle ear infections, headache, lightheadedness or dizziness, asthma, and nosebleeds. The following side effects have been identified during post-approval use of the SINUVA sinus implant. These events include implant migration, lack of efficacy, nasal pain, headache, and nosebleeds.
Tell your doctor if you have any side effects that bother you or don’t go away.
Risks related with the insertion and removal of SINUVA are similar to other endoscopic sinus procedures.
SINUVA is made from materials designed to soften over time and may fall out of the nose on its
own as polyps decrease or if you sneeze or blow your nose forcefully. The implant will be removed 90 days after placement or earlier at your doctor’s discretion.
Contact your doctor immediately if you have any changes in vision, excessive nasal bleeding, symptoms of infection or symptoms suggesting that the implant has moved, such as irritation or a choking sensation in the back of the throat.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
For important risk and use information, please see Full Prescribing Information for SINUVA.
Indication and Important Safety Information
See MoreINDICATION
SINUVA Sinus Implant is a prescription steroid-releasing implant indicated for the treatment of chronic rhinosinusitis with nasal polyps in adult patients 18 years or older who have had ethmoid sinus surgery.
IMPORTANT SAFETY INFORMATION
Who should not use SINUVA?
Do not use SINUVA if you are allergic to mometasone furoate or any ingredients of the implant.
What should I tell my doctor before receiving SINUVA?
Before you receive SINUVA, tell your doctor about all medical conditions you have including nasal/sinus problems (such as nasal ulcers or trauma), eye problems (such as glaucoma or cataracts), or any untreated fungal, bacterial, or viral infections.
What are the possible side effects of SINUVA?
Serious side effects of SINUVA can include:
-
Local nasal adverse reactions including nosebleed and injury to nerves or blood vessels in the nose/sinus.
-
Serious allergic reactions have happened in patients using mometasone furoate including rash, itching or swelling of the lips, face, tongue, and throat, and breathing problems. Call your doctor right away if you have any of these reactions.
-
Weakened immune system that may increase your risk of infections. Avoid contact with people who have contagious diseases such as chickenpox or measles. Call your doctor right away if you have been near someone with chickenpox or measles.
-
Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones and can cause tiredness, weakness, nausea and vomiting and low blood pressure. Talk to your doctor if steroid effects such as Cushing Syndrome and adrenal suppression appear.
The most common side effects of SINUVA in clinical studies were bronchitis, cold symptoms, middle ear infections, headache, lightheadedness or dizziness, asthma, and nosebleeds. The following side effects have been identified during post-approval use of the SINUVA sinus implant. These events include implant migration, lack of efficacy, nasal pain, headache, and nosebleeds.
Tell your doctor if you have any side effects that bother you or don’t go away.
Risks related with the insertion and removal of SINUVA are similar to other endoscopic sinus procedures.
SINUVA is made from materials designed to soften over time and may fall out of the nose on its
own as polyps decrease or if you sneeze or blow your nose forcefully. The implant will be removed 90 days after placement or earlier at your doctor’s discretion.
Contact your doctor immediately if you have any changes in vision, excessive nasal bleeding, symptoms of infection or symptoms suggesting that the implant has moved, such as irritation or a choking sensation in the back of the throat.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
For important risk and use information, please see Full Prescribing Information for SINUVA.